Direct-to-consumer (DTC) advertising of prescription drugs is a big business in the United States, as it is in New Zealand, the only other country in which this activity is legal. Anyone who watches TV for more than an hour or two a week will be more than familiar with the sorts of ads we are talking about, those that invariably end with an ominous list of potential side effects of the very drugs being pushed.
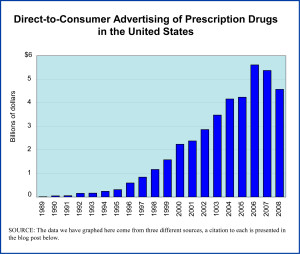
Until the late 1990s this advertising was quite limited by FDA regulations but in 1997 the FDA announced changes to those regulations (implemented in 1999) that freed up the pharmaceutical industry to start producing stylish ad campaigns for its most popular prescription drugs. The industry took full advantage, as the graph above clearly shows. Industry analysts suggest that the slowdown in spending starting in 2007 had more to do with the expiration of important brand name drugs (and their replacement with generics for which such spending is not done) than with the beginning of the recession.
Today’s market size is the amount spent by pharmaceutical companies on DTC prescription drug advertising in 2008.
Geographic reference: United States
Year: 2008
Market size: $4.57 billion
Source: For the data from 1989 through 2001: Francis B. Palumbo and C. Daniel Mullins, “The Development of Direct-to-Consumer Prescription Drug Advertising Regulations, Food and Drug Law Journal, Volume 57, Number 2, 2002. For the data from 2002 through 2005: Donahue Ph.D., Julie M., Marisa Cevasco, B.A. and Meredith B. Rosenthan, Ph.D., “A Decade of Direct-to-Consumer Advertising of Prescription Drugs,” The New England Journal of Medicine, August 16, 2007. For data from 2006 through 2008 the data are from Nielsen Media press releases.
Posted on January 20, 2012